Titanium and its alloys have become the cornerstone materials of modern dental implant restoration due to their excellent biocompatibility, mechanical properties, and osseointegration ability. This paper systematically describes the classification of titanium materials, their core properties, their wide application in the dental field (with implants as the focus), processing technologies and their impact, current challenges, and prospects such as new alloys, additive manufacturing, and surface nano-engineering, aiming to provide theoretical references for clinical practice and research.

1. Introduction
Titanium (Ti), the 22nd element in the periodic table, is a silver-white transition metal. Since the 1950s when Swedish scholar Per-Ingvar Brånemark discovered that titanium could form a direct, functional, and structural connection with bone tissue (known as “osseointegration”), titanium has opened a brilliant chapter in the biomedical field, especially in dental implantology. Its unique combination of properties perfectly meets the rigorous requirements of oral rehabilitation materials: excellent biocompatibility to avoid rejection, extremely high specific strength to withstand chewing forces, outstanding corrosion resistance against saliva erosion, and good osteoconductivity to ensure long-term stability. The success and predictability of dental implant treatments heavily depend on these core characteristics of titanium, making it the irreplaceable “king of biomaterials” in dental restorations, particularly for tooth loss rehabilitation.
2. Classification of Titanium and Titanium Alloys
Titanium materials used in the dental field can be classified into two main categories based on their composition and properties:
- 2.1 Commercially Pure Titanium (CP Ti):
- According to ASTM standards (e.g., F67), CP Ti is divided into Grades 1 to 4 based on the content of interstitial elements such as oxygen and iron.
- 特徴 Offers the best biocompatibility (most inert), excellent corrosion resistance, and good plasticity, making it easy to process. However, its strength (especially yield and fatigue strength) is relatively low.
- Dental Applications: Mainly used for implants, abutments, crown and bridge frameworks, and orthodontic brackets where strength demands are not extreme. CP Ti Grade 4 is the most widely used.
- 2.2 Titanium Alloys:
- By adding alloying elements (e.g., Al, V, Nb, Zr, Mo, Ta), the mechanical properties of titanium are enhanced.
- Representative Alloy – Ti-6Al-4V (Grade 5):
- The classic α+β phase titanium alloy (6% aluminum stabilizes the α phase, 4% vanadium stabilizes the β phase).
- 特徴 Much stronger than CP Ti (about twice the strength of CP Ti Grade 4), excellent fatigue properties, and good corrosion resistance. However, concerns exist over the potential biological toxicity of vanadium (V) and aluminum (Al) (despite limited clinical evidence), and its elastic modulus is still higher than that of bone.
- Other New/Dental-Specific Alloys:
- Ti-6Al-7Nb, Ti-5Al-2.5Fe: Designed to replace Ti-6Al-4V, avoiding vanadium for better biocompatibility.
- β-Type Titanium Alloys (e.g., Ti-13Nb-13Zr, Ti-12Mo-6Zr-2Fe – TMZF, Ti-35Nb-7Zr-5Ta): Utilize niobium (Nb), tantalum (Ta), zirconium (Zr), and molybdenum (Mo) as main alloying elements.
- 特徴 Lower elastic modulus (closer to bone, reducing stress shielding), higher fatigue strength and fracture toughness, excellent corrosion resistance, free from controversial elements (like V and Al), and superior biocompatibility. These are currently a research hotspot.
- Ti-Zr Alloys (e.g., Roxolid® – Ti-15Zr): Developed specifically for dentistry, offering higher strength than CP Ti and approaching that of Ti-6Al-4V, but with biocompatibility closer to pure titanium and moderate elastic modulus. Particularly suited for small-diameter implants.
Table 1: Comparison of Properties of Common Dental Titanium and Titanium Alloys
| Material Type | Typical Grade | Main Composition | メリット | Limitations | Main Dental Applications |
|---|---|---|---|---|---|
| Commercially Pure Ti | Grade 1 | >99% Ti | Best biocompatibility, excellent corrosion resistance, ductile | Lowest strength | Thin-walled parts, membranes |
| Grade 2 | >98.9% Ti | Good overall performance (balance of biocompatibility, corrosion resistance, strength, plasticity) | Moderate strength | Abutments, small restorations, brackets | |
| Grade 3 | >98.8% Ti | Higher strength than Grade 2 | Slightly less ductile than Grade 2 | Implants (some), abutments, frameworks | |
| Grade 4 | >98.6% Ti | Strongest CP Ti | Lower ductility than lower-grade CP Ti | Mainstream implants, abutments, archwires | |
| α+β Titanium Alloys | Ti-6Al-4V (Gr5) | Ti-6Al-4V | High strength, excellent fatigue resistance | Contains Al/V (potential bio-toxicity), high elastic modulus | Implants in high-load areas, connectors, surgical tools |
| Ti-6Al-7Nb | Ti-6Al-7Nb | Strength close to Gr5, no V (better biocompatibility) | Contains Al | Implants (Gr5 substitute) | |
| β-Type Alloys | Ti-13Nb-13Zr | Ti-13Nb-13Zr | Low elastic modulus, excellent biocompatibility, no Al/V | Complex processing | Implants (to mitigate stress shielding), orthodontic wires |
| Ti-12Mo-6Zr-2Fe (TMZF) | Ti-12Mo-6Zr-2Fe | Low elastic modulus, high strength, good corrosion resistance | Contains Fe (limited long-term bio data) | Implants, orthopedic implants | |
| Dental-Specific Alloy | Ti-15Zr (Roxolid®) | Ti-15Zr | Much stronger than CP4, close to Gr5, biocompatibility close to CP Ti | Higher cost | Small diameter/narrow ridge implants |
(Note: This table is a simplified comparison. Exact performance parameters should refer to material standards and manufacturer data. Elastic modulus: CP Ti ~100–110 GPa, Ti-6Al-4V ~110–115 GPa, β alloys ~55–85 GPa, cortical bone ~10–30 GPa)
3. Core Advantages of Titanium in Dentistry
Titanium and its alloys exhibit unparalleled advantages in dental applications:
- 3.1 Outstanding Biocompatibility and Corrosion Resistance:
- 生体適合性: Titanium can spontaneously form a dense, stable, and inert titanium dioxide (TiO₂) passive film in physiological environments. This film effectively isolates the release of metal ions into surrounding tissues, minimizing inflammation and immune rejection — a prerequisite for osseointegration. Numerous clinical studies and long-term follow-up data confirm titanium implants’ excellent tissue responses.
- Corrosion Resistance: The TiO₂ passive layer has strong resistance to body fluids (saliva, blood, interstitial fluid), fluorides (from toothpaste and mouthwash), and oral pH fluctuations (e.g., from acidic foods and drinks). Even during long-term service, issues like pitting, crevice corrosion, or stress corrosion cracking are extremely rare, ensuring the long-term chemical stability and safety of implants in the harsh oral environment.
- 3.2 High Strength-to-Weight Ratio (High Specific Strength):
Titanium has a significantly lower density (~4.5 g/cm³) than stainless steel (~8 g/cm³) and cobalt-chromium alloys (~8.5 g/cm³), yet its strength (especially that of titanium alloys) is comparable to or even exceeds these materials. This means that titanium restorations or implants can provide sufficient load-bearing capacity (withstand chewing forces, usually up to several hundred newtons) while being lighter in weight, thereby reducing the burden on supporting tissues and improving patient comfort. - 3.3 Excellent Osseointegration Capability:
This is the core advantage of titanium as an implant material. The TiO₂ layer on the surface of titanium is bioactive and can selectively adsorb proteins from blood and body fluids (such as fibrinogen and fibronectin), promoting the adhesion, proliferation, and differentiation of osteoblasts. Eventually, new bone tissue can directly deposit and tightly bond to the titanium surface, forming a direct structural and functional connection without a fibrous connective tissue interface. This direct bone-to-implant integration provides the biological basis for the long-term stable retention of the implant. - 3.4 Radiolucency and Aesthetic Advantages:
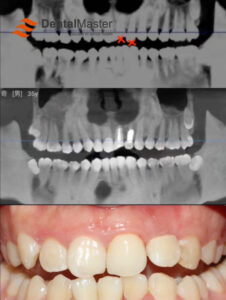
Radiolucency: Titanium has much lower X-ray attenuation than traditional metals like gold alloys and cobalt-chromium alloys. On radiographs (periapical films, panoramic films, CBCT), titanium implants appear as relatively low-density images, allowing clear visualization of the surrounding bone structure. This facilitates clinicians’ accurate assessment of osseointegration status, marginal bone level changes, and peri-implant health, making it key for long-term follow-up monitoring.
Aesthetic Advantage: For thin gingiva or superficially placed implants (especially in the anterior zone), traditional metal abutments (e.g., gold alloys) may cause a grayish discoloration through the gingiva, affecting aesthetics. Although titanium abutments can pose a similar risk, their gray tone is generally lighter and can be completely avoided by selecting all-ceramic abutments or titanium abutments with ceramic or zirconia coatings. As a material for abutments or frameworks, titanium also provides reliable support for all-ceramic restorations, enabling natural-looking aesthetic results.

4. Common Applications of Titanium in Dentistry
Titanium is widely used in various fields of dental restoration, implantation, orthodontics, and surgery:
4.1 Dental Implants:
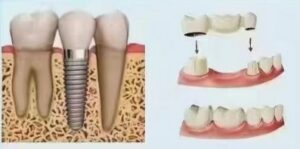
This is the most central and successful application of titanium in dentistry. Titanium implants serve as artificial tooth roots, surgically inserted into the jawbone to support superstructures (crowns, bridges, overdentures). Their designs (root-form, cylindrical, blade-form), surface treatments (SLA, SLActive, anodization, etc.), and connection methods (internal hex, Morse taper, platform switching) continue to evolve to meet various bone conditions, prosthetic needs, and aesthetic demands. The long-term success rate (over 10 years) often exceeds 95% under strict indications.
4.2 Abutments and Prosthetic Components:
Abutments: The key components connecting the implant platform to the superstructure. They can be made of titanium (CP Ti or alloy), zirconia, or a combination (titanium base + zirconia top). チタン製アバットメント are strong, reliable in connection with implants, and biocompatible, making them a mainstream choice—especially suitable for posterior regions or angled abutments.
Prosthetic Screws: Used to secure abutments or restorations to implants, usually made from high-strength titanium alloys (e.g., Ti-6Al-4V). They require extremely high fatigue resistance and dimensional precision.
Sleeves, Connectors: In complex prosthetic structures (such as bar-retained overdentures), titanium connectors provide stability and strength.
4.3 Orthodontic Archwires and Brackets:
Archwires: Beta titanium alloys (e.g., TMA – titanium-molybdenum alloy) are favored in fixed orthodontics due to their lower elastic modulus (softer, continuous forces), high elastic limit (large deformation range), good formability, and biocompatibility. Commonly used in the later stages for fine adjustments.
Brackets: Titanium metal brackets are mainly used for patients allergic to nickel, offering good biocompatibility and sufficient strength.
4.4 Surgical Tools and Instruments:
Titanium’s excellent strength, light weight, and corrosion resistance make it ideal for manufacturing high-precision, durable dental surgical instruments, such as implant surgical guides, osteotomes, needle holders, retractors, and implant carriers/wrenches. Its non-magnetic nature also suits special examination environments (e.g., MRI).
4.5 Crowns, Bridges, and Frameworks (Denture Frameworks):
Crown/Bridge Frameworks: Commercially pure titanium (mainly Grades 1 and 2) can be cast or CAD/CAM milled to fabricate crown or bridge substructures. Advantages include lightweight, good biocompatibility, and radiolucency. Disadvantages include casting difficulties (oxidation, shrinkage) and aesthetics inferior to all-ceramic (veneering porcelain is needed, and bonding to titanium is a key challenge).
Removable Denture Frameworks: Compared to traditional cobalt-chromium alloys, titanium frameworks significantly reduce weight, improve wearing comfort, enhance biocompatibility, have less impact on taste, and pose no allergy concerns. Commonly used in partial removable denture bases and clasps.

5. Titanium Processing and Surface Treatment: Key to Shaping Performance
The performance of titanium depends not only on its inherent properties but also heavily on its processing methods and surface condition—especially crucial for implant osseointegration.
5.1 Processing Techniques:
Cold Working: Such as rolling and drawing, used to produce titanium wires (orthodontic archwires), sheets (frameworks), and rods (machined abutments, implant blanks). Increases strength but reduces ductility.
Machining (Subtractive Manufacturing): The main method for manufacturing implants, abutments, and customized prosthetic components. Requires high-rigidity machines, specialized tools (carbide, diamond-coated), and coolant (strictly preventing oxidation). Extremely high precision is needed (micron-level), with relatively high costs. CAD/CAM technology is widely applied in this field for high-precision, efficient customization.
Casting: Used to make crown/bridge frameworks and denture structures. Must be performed in vacuum or inert gas (argon) environments to prevent oxidation and gas absorption (hydrogen, oxygen). Castings often have shrinkage, internal defects, and surface reaction layers (α-case), requiring post-treatment (sandblasting, acid etching, hot isostatic pressing – HIP) to improve performance.
Additive Manufacturing (3D Printing): Techniques like Selective Laser Melting (SLM) and Electron Beam Melting (EBM) allow complex titanium parts (custom abutments, frameworks, surgical guides, porous implants) to be fabricated directly from 3D digital models. These methods offer high design freedom, efficient material usage, and the ability to create intricate internal structures (e.g., biomimetic porous structures for bone ingrowth). It is one of the core directions for future development (see section 7 for details).
5.2 Surface Modification Technologies:
The micro- and nanoscale morphology, chemical composition, and wettability (hydrophilicity) of the implant surface have a decisive impact on protein adsorption, cell behavior (adhesion, proliferation, differentiation), and the final speed and quality of osseointegration. The main technologies include:
Mechanical Treatments:
- Machining/Grinding: Forms smooth or regularly textured surfaces. Used frequently in early applications, now mainly applied to the implant neck region (to reduce plaque attachment).
- Grit Blasting/Sandblasting: Surfaces are bombarded with alumina (Al₂O₃), titanium dioxide (TiO₂), or biocompatible ceramic particles (such as hydroxyapatite, HA) to create macroscopic roughness (micron scale), increasing surface area and mechanical interlocking ability. Often combined with acid etching.
Chemical Treatments:
- Acid Etching: Uses strong acids (such as mixed HCl/H₂SO₄, HF/HNO₃) to dissolve the titanium surface, forming complex porous morphology at micron or even nanoscale. Increases specific surface area and improves wettability. Forms the basis of many composite treatments.
- Alkali Heat Treatment: Titanium is immersed in NaOH solution then heated, forming a sodium titanate gel layer on the surface, which can transform into bone-like apatite in body fluids, significantly enhancing bioactivity.
Physicochemical Treatments:
- Sandblasted, Large-grit, Acid-etched (SLA®): First, sandblasting creates micron-scale roughness, followed by acid etching to remove blasting residues and add nanoscale structures. This is currently one of the most commercially successful, widely used, and clinically supported surface treatment technologies, significantly accelerating osseointegration and improving initial stability.
- Hydrophilic SLA (SLActive®): After SLA treatment, surfaces are stored/packaged under nitrogen protection or in saline solution to maintain high surface energy and hydrophilicity (contact angle near 0°). Hydrophilicity greatly promotes rapid spreading of blood and body fluids on the implant surface, accelerating fibrin matrix formation and osteoblast recruitment, achieving faster bone healing (weeks instead of months compared to traditional SLA).
- Anodic Oxidation: Applying voltage in an electrolyte allows controlled growth of a thick, porous TiO₂ film (which may include calcium and phosphate elements) on the titanium surface. Film thickness (which affects color interference for esthetic abutments), porosity, and composition can be precisely controlled, improving corrosion resistance, bioactivity, and (under specific conditions) antibacterial properties.
Coating Technologies:
- Hydroxyapatite (HA) Coating: Depositing a bioactive HA ceramic layer on titanium surfaces by methods such as plasma spraying (TPS). Intended to provide direct osteoconductive/osteoinductive interface. However, issues exist regarding coating adhesion and long-term stability (possible degradation and delamination), and it is less widely used than SLA.
- Other Bioactive Molecule Coatings: Such as bone morphogenetic proteins (BMP), peptides (RGD), growth factors, etc., currently in research stages, aimed at providing more active biological signals on the surface.
5.3 Effects of Surface Treatments on Biological Responses and Clinical Performance:
- オッセオインテグレーションの促進: Roughened (SLA, SLActive) and bioactivated (HA coating, alkali heat treatment) surfaces significantly shorten the implant healing period (from 3–6 months on traditional smooth surfaces to 3–6 weeks or even less), allowing immediate or early loading and improving patient satisfaction.
- Enhanced Biomechanical Locking: Micro/nanoscale rough surfaces increase bone-to-implant contact area (BIC%) and form mechanical interlocking structures, greatly improving initial implant stability and long-term load-bearing capacity.
- Impact on Soft Tissue Attachment: Surface characteristics (such as smoothness and hydrophilicity) of the transgingival part (neck) of the implant are crucial for epithelial cell and fibroblast attachment, forming a good soft tissue seal (biologic width), which is key to preventing bacterial invasion and maintaining peri-implant health.
- Potential Risks: Excessively rough or microcracked surfaces may increase bacterial colonization risk, requiring higher control standards for peri-implantitis. The long-term stability of coatings (such as HA) still needs more evidence.

6. Challenges and Limitations
Although titanium has achieved great success in dentistry, some challenges remain:
6.1 Raw Material and Processing Costs:
- Extraction and refining of titanium ores (rutile, ilmenite) via the Kroll or Hunter processes are energy-intensive and complex, resulting in raw material costs significantly higher than stainless steel or cobalt-chromium alloys.
- Processing titanium (casting, machining, 3D printing) is difficult, requiring specialized equipment, strict environmental controls (inert gas protection), and highly skilled workers, further increasing manufacturing costs. This makes final titanium-based dental products (especially implants and customized restorations) relatively expensive.
6.2 Allergies or Rare Sensitivities:
- Titanium is generally considered to have extremely low allergenicity. However, rare but documented clinical cases report delayed-type hypersensitivity (type IV allergic reaction) in a very small number of individuals to titanium metal or alloy elements (e.g., aluminum, vanadium, nickel impurities).
- Symptoms may include persistent peri-implant inflammation, mucosal redness and swelling, pain, and even osseointegration failure.
- Diagnosis is difficult due to a lack of highly specific and sensitive standardized titanium allergy tests (e.g., patch test interpretation is controversial).
- Although incidence is very low (much lower than nickel allergy), management of suspected patients (e.g., switching to zirconia implants) and mechanism research remain important clinical topics.
6.3 Wear and Electrochemical Corrosion in Mixed Metal Environments:
- Fretting/Corrosion: Implant systems include multiple metal interfaces (e.g., abutment screws and implant internal threads, abutment-implant connections). Functional micro-movements (chewing forces, occlusion) cause fretting wear, which damages protective oxide films and exposes fresh metal.
- Galvanic Corrosion: When different metals exist in the oral cavity (e.g., titanium implants + gold crowns, stainless steel orthodontic wires + titanium brackets) and are connected by saliva (electrolyte), potential differences cause accelerated corrosion dissolution of the metal with the lower potential (anode, e.g., titanium).
- Even different grades or surface states of titanium may have slight potential differences.
- Crevice Corrosion: In tightly fitted but not absolutely sealed metal connections (e.g., abutment-implant interface), reduced oxygen concentration, lower pH, and chloride ion accumulation in crevices may induce localized corrosion.
- その結果: These corrosion processes may weaken mechanical strength, release metal ions/particles into surrounding tissues, possibly triggering local or systemic reactions (e.g., allergy, inflammation), and affect long-term implant stability and lifespan. Strict control of material compatibility, optimized connection designs (e.g., Morse taper seals), and improved manufacturing precision to reduce micromotion are key countermeasures.
7.Future Development and Innovation
To overcome existing challenges and further enhance performance, research on titanium-based dental materials focuses on the following directions:
7.1 Development of New Titanium-Based Alloys:
- Optimization of Aluminum- and Vanadium-Free Alloys: Continue promoting and optimizing alternative alloys such as Ti-6Al-7Nb, and deeply develop β-type titanium alloys with superior performance and better biocompatibility (e.g., Ti-Nb-Zr-Ta system alloys). The goal is to achieve ultra-low elastic modulus close to bone tissue, higher fatigue limits, and excellent corrosion resistance.
- Titanium-Zirconium Alloys (Ti-Zr): Alloys represented by Roxolid® (Ti-15Zr) have been successfully commercialized. Their strength (>850 MPa) is much higher than that of commercially pure (CP) titanium (~550 MPa) and close to Ti-6Al-4V (~900 MPa), while maintaining biocompatibility comparable to pure titanium and excellent osseointegration ability. Future directions include optimizing Zr content, exploring Ti-Zr alloys combined with other elements (such as Nb, Ta), and expanding their application to a wider range of implant restoration components (e.g., abutment screws, personalized abutments).
- Low-Modulus High-Toughness Alloys: Designing alloys with nanostructures or special phase compositions to break through the traditional strength-modulus limitations of conventional alloys.
7.2 Deep Integration of Additive Manufacturing (3D Printing) and Digital Dentistry:
- Customized Complex Structures: SLM/EBM technologies can precisely manufacture complex geometries that are impossible with traditional processes, such as highly biomimetic, internally porous gradient-structured personalized implants (promoting bone ingrowth and vascularization), anatomically matched personalized abutments, and bone augmentation scaffolds (GBR membranes, titanium meshes).
- Topology Optimization: Using computer algorithms, design implant or restorative scaffold structures with optimal material distribution, minimal weight, and the most uniform stress distribution based on force analysis.
- Integrated Workflow: 3D printing seamlessly integrates into digital dentistry processes (oral scanning/CBCT data acquisition → CAD design → CAM printing), achieving fully traceable, efficient, and precise manufacturing of restorations/implant guides/implants. Reducing costs and improving accessibility are key for wider adoption.
7.3 Application of Nanotechnology in Titanium Surface Engineering:
Nanostructure Construction: Through chemical (e.g., double acid etching, alkali-heat treatment), physical (e.g., laser ablation, ion beam), or electrochemical (e.g., anodization) methods, precisely build nanotubes, nanopores, nanoparticles, nanowrinkles, and other nanostructures on titanium surfaces. These nanoscale features more effectively mimic the extracellular matrix (ECM), directly regulating cell behaviors (osteoblasts, stem cells) including adhesion, spreading, migration, proliferation, and differentiation, and even influencing gene expression.
Functionalized Nano Coatings:
Antibacterial Coatings: Load antibacterial agents such as silver nanoparticles (AgNPs), zinc oxide nanoparticles (ZnO NPs), antimicrobial peptides (AMPs), chitosan, etc., to impart long-lasting antibacterial properties to the surface and actively prevent peri-implantitis.
Osteogenic/Angiogenic Promoting Coatings: Load growth factors (e.g., BMP-2, VEGF), bioactive ions (e.g., Sr²⁺, Mg²⁺, Li⁺), or small molecule drugs to precisely regulate the local microenvironment, promoting rapid and high-quality bone regeneration and vascularization.
Smart Responsive Coatings: Design smart coatings that respond to changes in the oral environment (such as pH drop or bacterial enzyme activation) to release antibacterial or anti-inflammatory drugs for on-demand treatment.
Improving Long-Term Surface Stability: Study the stability, degradation behavior, and biological response of nanostructures during long-term service.
結論
Titanium and titanium alloys have established their core status in modern dentistry, especially dental implantology, due to their unparalleled biocompatibility, excellent mechanical properties (particularly high strength-to-weight ratio), outstanding corrosion resistance, and unique ability to promote osseointegration. From implants serving as artificial tooth roots, to abutments for connection and transition, to supporting scaffolds and efficient orthodontic tools, titanium materials run through all aspects of dental restoration, greatly advancing oral function reconstruction and aesthetic restoration.
Despite challenges such as cost, rare allergies, and corrosion in mixed-metal environments, advances in materials science and manufacturing technology continue to open new pathways. Novel titanium-based alloys (such as high-strength Ti-Zr alloys and low-modulus β-type alloys) aim to enhance performance while ensuring biosafety; additive manufacturing (3D printing) has ushered in a new era of highly personalized, complex-structured restorations and implant manufacturing; and nanostructured surface engineering enables unprecedented precision in controlling the material-biological interface, endowing titanium surfaces with antibacterial, osteogenic, and even smart responsive functions.
Looking ahead, titanium-based materials will remain the “gold standard” and a key innovation carrier in dental implant restoration. Through continuous material innovation, advanced manufacturing processes, and precise surface design, the application of titanium in dentistry will move toward being more minimally invasive, faster, more durable, more personalized, and safer, ultimately providing patients with oral restoration solutions that excel in both function and aesthetics, with improved long-term prognosis, significantly enhancing patients’ quality of life. The legendary story of titanium in oral medicine is far from over and continues to write new brilliant chapters.
References
Brånemark, P. I., Adell, R., Breine, U., Hansson, B. O., Lindström, J., & Ohlsson, A. (1977). Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scandinavian Journal of Plastic and Reconstructive Surgery, 11(3), 81–100.
(Introduced the concept of osseointegration and established the biological foundation of titanium implants.)
ASTM International. (2019). ASTM F67-13: Standard specification for unalloyed titanium for surgical implant applications. ASTM.
ASTM International. (2020). ASTM F136-13: Standard specification for wrought titanium-6aluminum-4vanadium ELI alloy for surgical implant applications. ASTM.
(Defines medical-grade standards for commercially pure titanium and Ti-6Al-4V alloy.)
Buser, D., Broggini, N., Wieland, M., et al. (2004). Enhanced bone apposition to a chemically modified SLA titanium surface. Journal of Dental Research, 83(7), 529–533.
(Classic study demonstrating accelerated osseointegration via SLA surface modification.)
Chiapasco, M., Casentini, P., & Zaniboni, M. (2012). Bone augmentation procedures in implant dentistry. International Journal of Oral & Maxillofacial Implants, 27(Suppl), s183–s203.
(Clinical data on Ti-15Zr alloy (Roxolid®) usage in narrow diameter implants.)
Kuroda, D., Niinomi, M., Morinaga, M., et al. (1998). Design and mechanical properties of new β type titanium alloys for implant materials. Materials Science and Engineering: A, 243(1-2), 244–249.
(Demonstrates the biomechanical advantages of low elastic modulus β-type titanium alloys.)
Van Noort, R. (2012). The future of dental devices is digital. Dental Materials, 28(1), 3–12.
(Reviews the prospects of digital manufacturing technologies such as 3D printing for dental implants.)
Mendonça, G., Mendonça, D. B., Aragão, F. J., & Cooper, L. F. (2008). Advancing dental implant surface technology—From micron-to nanotopography. Biomaterials, 29(28), 3822–3835.
(Discusses how nanoscale surface topographies modulate cellular behavior.)
Siddiqi, A., Payne, A. G., De Silva, R. K., & Duncan, W. J. (2011). Titanium allergy: A literature review. International Journal of Oral & Maxillofacial Implants, 26(4), 743–750.
(Systematic review on titanium allergy epidemiology and diagnostic challenges.)
Reclaru, L., & Meyer, J. M. (1994). Electrochemical corrosion in restorative dentistry. Dental Clinics of North America, 38(2), 319–332.
(Explores electrochemical corrosion mechanisms in the oral environment with multiple metals.)
Pjetursson, B. E., Thoma, D., Jung, R., et al. (2012). A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clinical Oral Implants Research, 23(Suppl 6), 22–38.
(Provides evidence-based data on implant survival rates over 10 years.)